An extensive study of the product application and services conducted by subject matter experts assessing the Hemodynamic Monitoring Devices market will help product owners to make a wise decision.
New York, United States – June 1, 2020 /MarketersMedia/ —
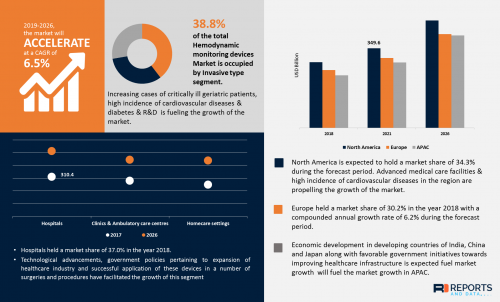
The global Hemodynamic Monitoring Devices Market was valued at USD 838.2 million in 2018 and is expected to reach USD 1.39 billion by the year 2026, at a CAGR of 6.2%. The measurement or estimation of hemodynamic variables such as blood pressure, blood flow, cardiac contractility, cardiac preload, and cardiac afterload plays a crucial role in the monitoring and diagnostic procedures especially in the intensive care unit or in case of patients having major surgery. Hemodynamic monitoring devices facilitate the accurate assessment of blood flow from inside the arteries, heart, and veins. Hemodynamic monitoring plays a fundamental role in the management of acutely ill patients.
Increasing cases of critical illness in geriatric patients, high incidence of cardiovascular diseases & diabetes, rise in demand for hemodynamic monitoring devices, increasing digitalization in hospital systems, technological advancements & increased funding scenario in research & development are key factors contributing to high CAGR of hemodynamic monitoring devices market during forecast period.
This report covers the recent COVID-19 incidence and its impact on Hemodynamic Monitoring Devices Market. The pandemic has widely affected the economic scenario. This study assesses the current landscape of the ever-evolving business sector and the present and future effects of COVID-19 on the market.
To request a sample or speak to an expert before you buy the report, click on the link below@ https://www.reportsanddata.com/sample-enquiry-form/2122
Key participants include Edwards Lifesciences Corporation, ICU Medical, Inc., LiDCO Group Plc., Drager Medical GmbH, Cheetah Medical, Hemo Sapiens, Inc., GE Healthcare, Schwarzer Cardiotek GmbH, Teleflex Incorporated, Siemens, Osypka Medical GmbH, PULSION Medical Systems SE, Deltex Medical Group Plc., Tensys Medical, Inc., McKessen, and Philips Medical.
Invasive hemodynamic monitoring is one of the major competencies required for critical care nurses. In clinical routine, it is commonly performed during high-risk surgery and in intensive care medicine. Continuous assessment of the clinical conditions of the cardiovascular system is essential for critically ill patients to diagnose and manage complex medical conditions. According to WHO, 17.9 million people die each year from cardiovascular diseases, which is an estimated 31% of all deaths worldwide. According to data, the cases of cardiovascular diseases are increasing in developing countries, which is expected to boost the market during the forecast period.
For the purpose of this report, Reports and Data has segmented the Hemodynamic Monitoring devices market on the basis of Product type, type, End-use, and region:
Product (Revenue, USD Million; 2016–2026)
• Monitors
• Disposables
Type (Revenue, USD Million; 2016–2026)
• Invasive
• Minimally invasive
• Non-invasive
End – Use (Revenue, USD Million; 2016–2026)
• Hospitals
• Clinics & Ambulatory Care Centres (ACCs)
• Homecare Settings
• Others
Grab this report at an amazing discount here @ https://www.reportsanddata.com/discount-enquiry-form/2122
Regional Outlook (Revenue in USD Million; 2016–2026)
• North America (United States, Canada and Mexico)
• Europe (Germany, France, UK, Russia and Italy)
• Asia-Pacific (China, Japan, Korea, India and Southeast Asia)
• South America (Brazil, Argentina, Colombia)
Further key findings from the report suggest
• The global Hemodynamic Monitoring devices market is forecasted to grow with a CAGR of 6.5% due to high incidence of cardiovascular diseases and diabetes
• The non-invasive type segment is forecasted to register the highest CAGR of 7.2%. Non-invasive systems allow the in-house assessment of hemodynamic statistics without compromising on the accuracy and reliability thereby increasing their demand during the forecast period
• The disposables segment dominated the market in terms of revenue generation. Furthermore, the monitoring devices market is expected to witness modest growth
• Tandem Mass Spectrometry segment dominated with a market share of 40.0% in 2018 and is expected to maintain its lead due to its increasing use in combination with liquid chromatography which aids in overcoming challenges associated with traditional techniques in Hemodynamic Monitoring devices.
Access Full Report@ https://www.reportsanddata.com/report-detail/hemodynamic-monitoring-devices-market
Thank you for reading our report. For further details or to inquire about customization, please let us know and we will offer you the report as per your needs.
About Us:
Our in-house experts assist our clients with advice based on their proficiency in the market that helps them in creating a compendious database for the clients. Our team offers expert insights to clients to guide them through their business ventures. We put in rigorous efforts to keep our clientele satisfied and focus on fulfilling their demands to make sure that the end-product is what they desire. We excel in diverse fields of the market and with our services extending to competitive analysis, research and development analysis, and demand estimation among others, we can help you invest your funds in the most beneficial areas for research and development. You can rely on us to provide every significant detail you might need in your efforts to make your business flourish.
Contact Info:
Name: John Watson
Email: Send Email
Organization: Reports And Data
Address: 40 Wall St. 28th floor New York City, NY 10005 United States
Phone: +1-212-710-1370
Website: https://www.reportsanddata.com/
Source: MarketersMedia
Release ID: 88959886






